

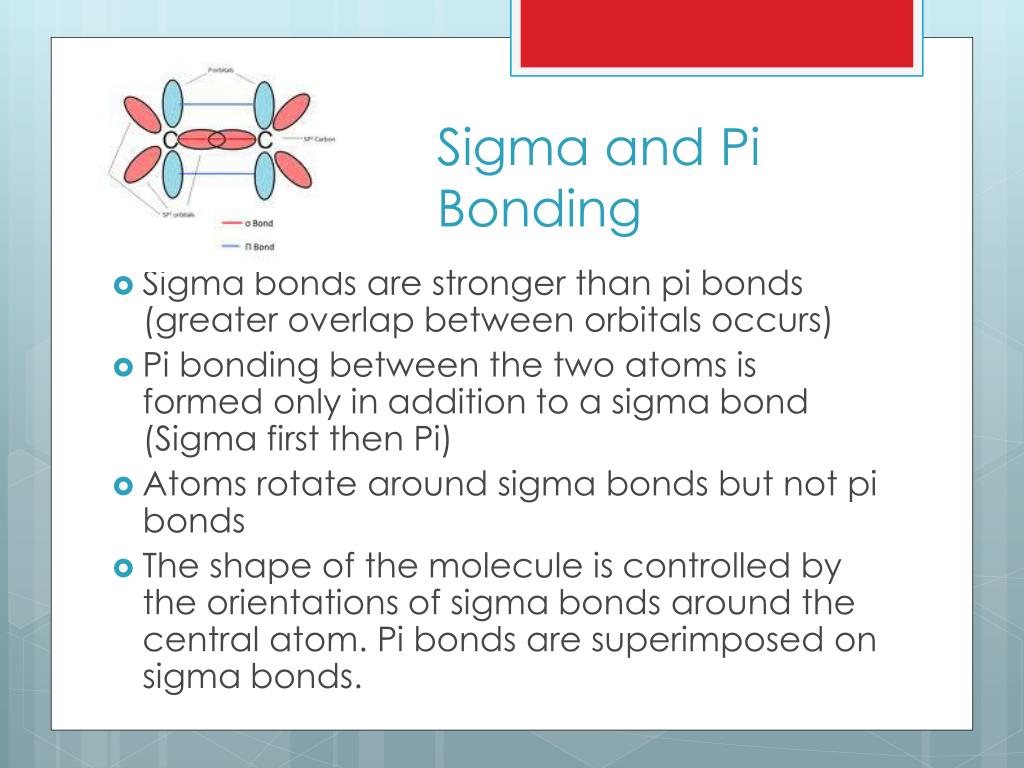
From the perspective of quantum mechanics, this bond's weakness is explained by significantly less overlap between the component p-orbitals due to their parallel orientation. The C-C double bond, composed of one sigma and one pi bond, has a bond energy less than twice that of a C-C single bond, indicating that the stability added by the pi bond is less than the stability of a sigma bond. Pi bonds are usually weaker than sigma bonds. Properties Two p-orbitals forming a π-bond. This latter mode forms part of the basis for metal-metal multiple bonding. One common form of this sort of bonding involves p orbitals themselves, though d orbitals also engage in pi bonding. The Greek letter π in their name refers to p orbitals, since the orbital symmetry of the pi bond is the same as that of the p orbital when seen down the bond axis.
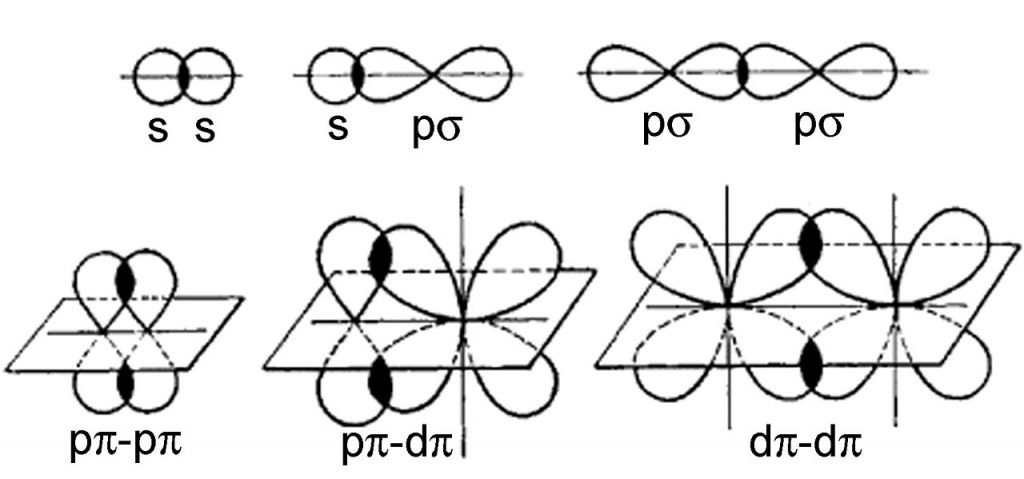
Pi bonds can form in double and triple bonds but do not form in single bonds in most cases. This plane also is a nodal plane for the molecular orbital of the pi bond.

Each of these atomic orbitals has an electron density of zero at a shared nodal plane that passes through the two bonded nuclei. In chemistry, pi bonds ( π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. JSTOR ( February 2013) ( Learn how and when to remove this template message)Įthylene (ethene), a small organic molecule containing a pi bond, shown in green.Unsourced material may be challenged and removed. Please help improve this article by adding citations to reliable sources. The orbitals would not be able to overlap, so the connection between the atoms would be lost.This article needs additional citations for verification. If one atom turns with respect to the other, the p orbital would have to stretch to maintain the connection. A s orbital is not affected when the atom at one end of the bond is rotated with respect to the other. In other words, there are more nodes in the higher-energy orbitals than in the lower-energy ones.Īn important consequence of the spatial distribution or "shape" of a p orbital is that it is not symmetric with respect to the bond axis. Just as the sigma-bonding orbitals display progressively shorter wavelengths along the bonding axis as they go to higher energy, so do the pi bonding orbitals. There will be both bonding and antibonding combinations. The two p orbitals orthogonal to the bond axis can engage in p bonding. In a main group diatomic species like dinitrogen, one p orbital lying along the bond axis can engage in s bonding. A similar picture could be shown for the other set of p orbitals. The second picture shows the result of the constructive (or destructive) interference. The illustration above is for one set of p orbitals that are orthogonal to the bond axis. the electron density is found above and below the bond axis.the resulting orbitals contain nodes along the bond axis.parallel p orbitals can overlap to produce bonding and antibonding combinations.They could also make an out-of-phase combination, as shown below. They can make an in-phase combination, as shown below. They can be close enough to each other to overlap, although they do not overlap as strongly as orbitals lying along the bond axis.
They would approach each other side by side, above and below the bond axis between the two atoms. Parallel, but not collinear, p orbitals can also interact with each other. \)Įarlier, we saw that p orbitals that lie along the same axis can interact to form bonds.


 0 kommentar(er)
0 kommentar(er)
